
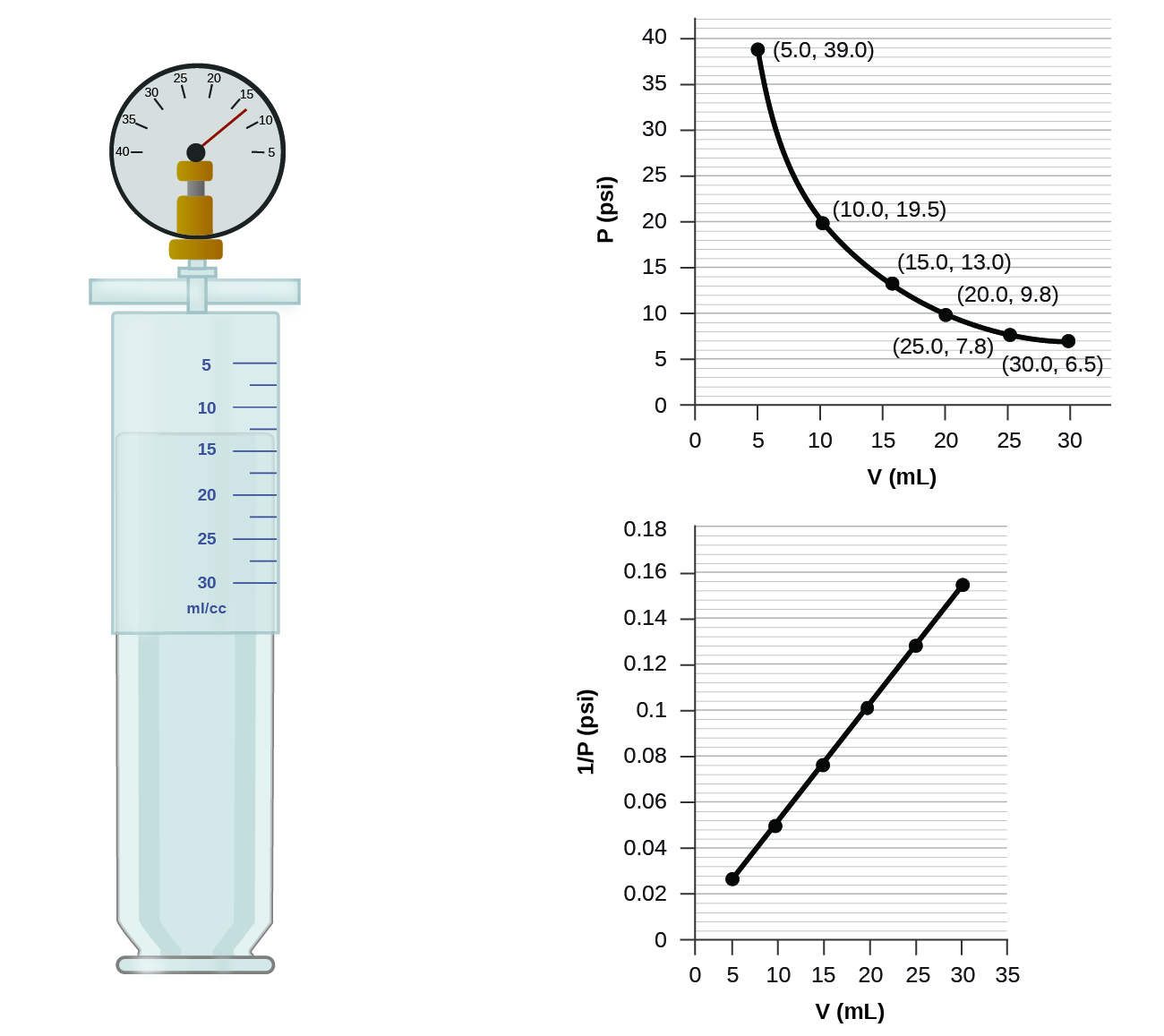
An example of experimental pressure-temperature graph is shown for a sample of air under these conditions in Figure 2.4.3. This relationship between temperature and pressure is observed for any sample of gas confined to a constant volume. As the gas is heated, the pressure of the gas in the sphere increases. The effect of temperature on gas pressure: When the hot plate is off, the pressure of the gas in the sphere is relatively low. If we heat the sphere, the gas inside gets hotter (Figure 2.4.2) and the pressure increases.įigure 2.4.2. Since the container is rigid and tightly sealed, both the volume and number of moles of gas remain constant. If the container is cooled, the gas inside likewise gets colder and its pressure is observed to decrease. Imagine filling a rigid container attached to a pressure gauge with gas and then sealing the container so that no gas may escape. Pressure and Temperature: Amontons’s /Gay-Lussac’s Law The launch of the latter was reportedly viewed by 400 000 people in Paris. When the hydrogen-filled balloon depicted in (a) landed, the frightened villagers of Gonesse reportedly destroyed it with pitchforks and knives. In 1783, the first (a) hydrogen-filled balloon flight, (b) manned hot air balloon flight, and (c) manned hydrogen-filled balloon flight occurred. We will consider the key developments in individual relationships (for pedagogical reasons not quite in historical order), then put them together in the ideal gas law.įigure 2.4.1. Eventually, these individual laws were combined into a single equation-the ideal gas law -that relates gas quantities for gases and is quite accurate for low pressures and moderate temperatures.

Although their measurements were not precise by today’s standards, they were able to determine the mathematical relationships between pairs of these variables (e.g., pressure and temperature, pressure and volume) that hold for an ideal gas-a hypothetical construct that real gases approximate under certain conditions. During the seventeenth and especially eighteenth centuries, driven both by a desire to understand nature and a quest to make balloons in which they could fly (Figure 2.4.1), a number of scientists established the relationships between the macroscopic physical properties of gases, that is, pressure, volume, temperature, and amount of gas.


 0 kommentar(er)
0 kommentar(er)
